Background: Diagnosis and risk prediction of thrombosis is challenging due to nonspecific symptoms, diverse risk factors, symptom overlap with other conditions and the variability in thrombosis presentations. Little is known on the impact of thrombosis on the plasma proteome across the spectrum of its clinical representations.
Objective: The aim of this study was to explore the plasma proteomic landscape across multi-center thrombosis cohorts. The focus was on identifying protein signatures representative of thrombosis in general or among patients at risk of thrombosis, those with a history of thrombosis, those at acute phase and those with COVID-19 associated thrombosis.
Method: Using state-of-the-art mass spectrometry-based proteomics, we analyzed 420 plasma proteome samples both cross-sectional and longitudinal from 211 patients, including those at risk of thrombosis, in the acute thrombotic phase (cerebral venous sinus thrombosis patients) and with COVID-19-associated thrombosis (Figure 1). Linear models and unsupervised co-expression clustering were used to explore common and distinct protein signatures among the cohorts.
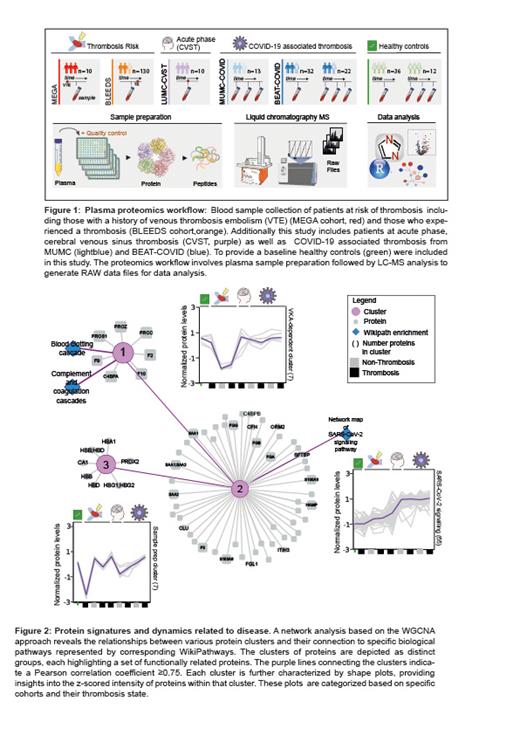
Results: Across the analyzed samples 475 proteins were quantified, with 86% showing a CV below 30%, and we found a strong correlation between protein levels quantified by MS and ELISA. Plasma proteomic alterations exhibited variation across cohorts, with the most pronounced changes observed in COVID-19 patients. While co-expression analysis unveiled distinct protein clusters associated with SARS-CoV-2 signaling pathways (CRP, SAA1) and Vitamin K-dependent (VKA) coagulation proteins (F9, F2, F10, PROS, PROC, PROZ, C4BPA), we did not identify a specific protein cluster related to thrombosis (Figure 2). Notably, VKA-therapy was associated with decreased abundance of VKA-dependent coagulation proteins, with F10 and PROZ showing significant alterations between patients who experienced a thrombosis compared to healthy controls. We observed an inverse correlation between the International Normalized Ratio and abundance of VKA-dependent coagulation proteins, with an increased correlation of F2 and F9 in patients who experienced a thrombotic event. Additionally, our analysis also highlighted potential variations in multi-center cohorts during sample handling, emphasizing the importance of standardized protocols in ensuring the reliability and comparability of results.
Conclusion: Using a comprehensive MS-based proteomics approach, we identified clear plasma proteomic signatures associated with VKA-treatment and COVID-19 , but no distinct thrombosis signature across multi-center thrombosis cohorts.
Disclosures
Eikenboom:CSL Behring: Research Funding.